Nunawa (amfani da aka yarda): A cikin 2019, FDA ta amince da ita don maganin samu, rashin lafiyar sha'awar jima'i na gaba ɗaya (HSDD) a cikin matan da suka riga sun yi aure lokacin da yanayin ya haifar da damuwa kuma ba saboda wasu yanayi na likita / tabin hankali ko illa na miyagun ƙwayoyi ba.
Tsarin Aiki
PT-141 shine melanocortin agonist mai karɓa (mafi mahimmanci MC4 receptor) wanda ke daidaita sha'awar jima'i ta hanyoyin tsarin juyayi na tsakiya.
Ba kamar PDE5 masu hanawa (misali, sildenafil), wanda yafi rinjayar tasoshin jini, PT-141 yana aiki a tsakiya don rinjayar sha'awar jima'i da tashin hankali.
Pharmacology & Dosing
Gudanarwa: Allurar subcutaneous, kamar yadda ake buƙata (kan buƙata).
Adadin da aka yarda: 1.75 MG sc
Pharmacokinetics:
Tmax ≈ ~ 60 minutes
t½ ≈ 2-3 hours
Tasirin na iya ɗaukar awoyi da yawa, a wasu rahotanni har zuwa ~16 hours.
Ingantattun Magunguna (Gwaji na Mataki na III - SAKE CONNECT, makonni 24, RCTs)
Ƙarshen farko:
Fihirisar Ayyukan Jima'i na Mata-Yankin Sha'awa (FSFI-D)
Ma'aunin Matsalolin Jima'i (FSDS-DAO)
Mahimmin sakamako (ilimin da aka tattara 301 + 302):
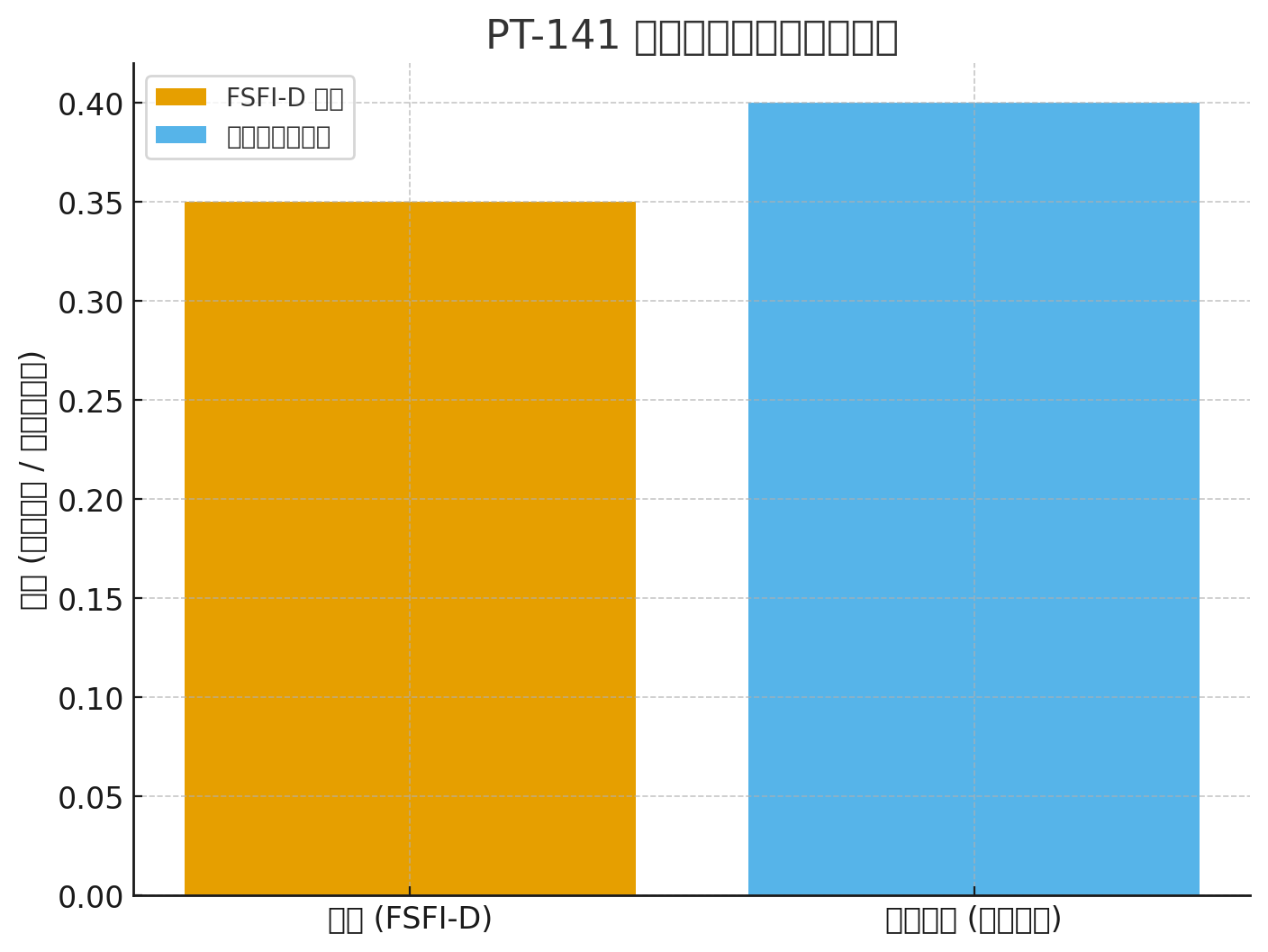
Inganta FSFI-D: +0.35 vs placebo (P <0.001)
Rage maki FSDS-DAO: -0.33 vs placebo (P <0.001)
Sauran abubuwan ƙarshe: Sakamakon goyan baya (ƙididdigar aikin jima'i, gamsuwar da aka ba da rahoton haƙuri) yana da kyau, amma abubuwan jima'i masu gamsarwa (SSEs) ba koyaushe suna nuna bambance-bambance masu mahimmanci ba.
Abubuwan da ba su da kyau (mafi yawan rahotanni a cikin gwaji)
Na kowa (≥10%):
Nausea (~ 30-40%; har zuwa ~ 40% an ruwaito a gwaji)
Fitowa (≥10%)
Ciwon kai (≥10%)
Tasirin zuciya:
Ƙaruwa na wucin gadi a cikin hawan jini kuma an lura da canje-canje a cikin bugun zuciya, yawanci ana warwarewa a cikin 'yan sa'o'i.
Contraindicated ko amfani da taka tsantsan a cikin marasa lafiya da hauhawar jini mara sarrafa ko cututtukan zuciya.
Hanta: Rare rahotanni na haɓakar enzyme hanta na wucin gadi; Rahotanni na lokuta da ba kasafai ba suna nuna yiwuwar raunin hanta mai tsanani, amma ba na kowa ba.
Tsaro na Dogon Lokaci (Nazarin Tsawaitawa)
Wani binciken fadada lakabin mako-mako 52 ya sami ci gaba a cikin sha'awar ba tare da sabbin siginonin aminci ba.
Bayanan martabar aminci na dogon lokaci ana la'akari da gabaɗaya da kyau, tare da manyan batutuwan jurewa har yanzu suna da illa na ɗan gajeren lokaci kamar tashin zuciya.
Mabuɗin Bayanan Amfani
Yawan jama'a da aka amince da shi yana da iyaka: Ga matan da suka riga suka yi hailar kawai waɗanda suka samu, HSDD gabaɗaya.
Ba a yarda da shi ba ga maza (ED ko ƙarancin sha'awar maza yana ci gaba da bincike).
Binciken aminci yana da mahimmanci: hawan jini, cututtukan zuciya, da tarihin hanta ya kamata a tantance kafin a ba da izini.
Takaitaccen Bayanin Saurin
Amincewar FDA: 2019 (Vyleesi).
Kashi: 1.75 MG allurar subcutaneous, akan buƙata.
PK: Tmax ~ 60 min; t½ 2-3 h; tasiri har zuwa ~ 16 h.
Inganci (Mataki na III, wanda aka haɗa):
FSFI-D: +0.35 (P<.001)
FSDS-DAO: -0.33 (P<.001)
Abubuwan da ba su da kyau:
Nausea: har zuwa ~ 40%
Ruwa: ≥10%
Ciwon kai: ≥10%
An lura da BP na wucin gadi.
Teburin Kwatanta & Hotuna (Taƙaitaccen)
| Nau'in Nazari / Data | Ƙarshen Ƙarshen / Auna | Darajar / Bayani |
|---|---|---|
| Mataki na III (301+302) | FSFI-D (yankin sha'awa) | +0.35 vs placebo (P <0.001); FSDS-DAO -0.33 |
| Abubuwan da ba su dace ba | Tashin zuciya, firgita, ciwon kai | Tashin zuciya ~ 30-40% (max ~ 40%); ragewa ≥10%; ciwon kai ≥10% |
Lokacin aikawa: Satumba-30-2025